by Dr. Yashashwini Reddy | Sep 6, 2025
Current Good Manufacturing Practices (cGMP) in Pharmaceutical Industries Current Good Manufacturing Practices (cGMP) are the regulatory standards enforced by agencies like the USFDA, WHO, and EMA to ensure that pharmaceutical products are consistently produced and...

by Dr. Yashashwini Reddy | Aug 18, 2025
✅ 30 Common Ways to Avoid the Most Frequent GMP Errors Follow approved SOPs strictly. Ensure proper documentation – never backdate or predate entries. Maintain good record-keeping practices (ALCOA+ principles). Always perform line clearance before starting new...
by Dr. Yashashwini Reddy | May 5, 2025
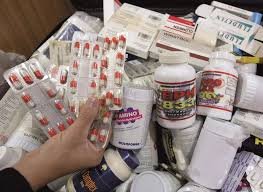
The quality of pharmaceutical products is crucial for ensuring their safety, efficacy, and reliability. When pharmaceuticals are substandard, they pose significant health risks, including treatment failures, adverse reactions, and even death. Various factors can...

by Dr. Yashashwini Reddy | Apr 29, 2025
Pharmaceutical compliance plays a crucial role in ensuring the overall quality of medicinal products. Compliance in the pharmaceutical industry refers to adherence to regulations, laws, and guidelines set by governing bodies such as the U.S. Food and Drug...
by Dr. Yashashwini Reddy | Nov 28, 2024
Why Data Integrity Matters in Pharmaceuticals: Insights from Patients and Regulators In pharmaceuticals, “data integrity” ensures data remains accurate, consistent, and reliable throughout its lifecycle. It forms the backbone of quality assurance in...






