by Dr. Yashashwini Reddy | Aug 18, 2025
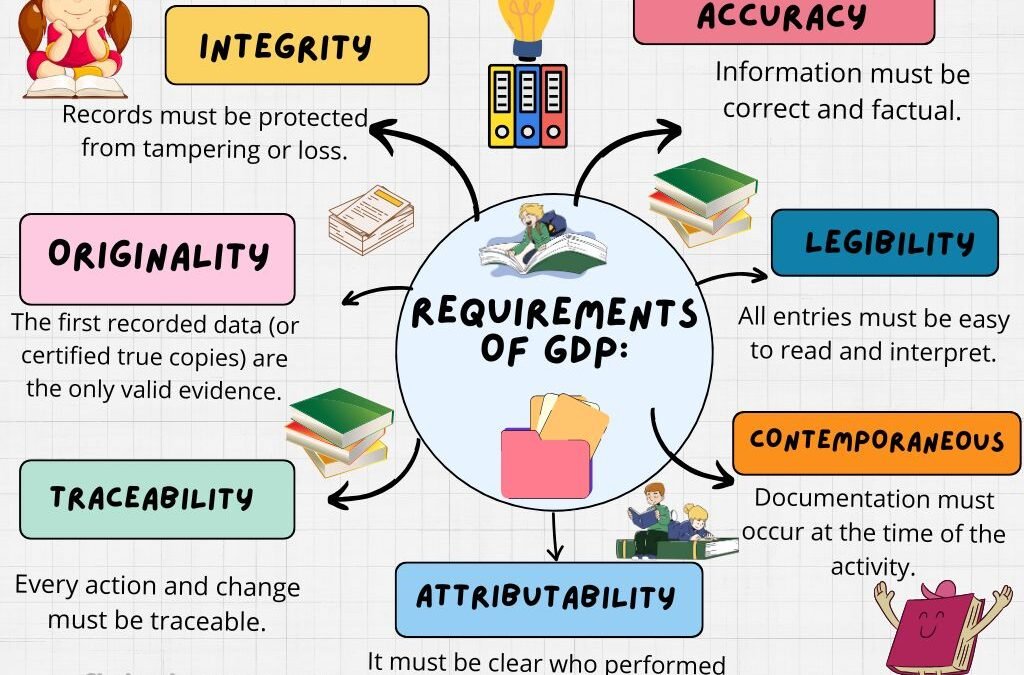
Requirements for Good Documentation Practice (GDP) Legibility All entries should be clear, readable, and permanent (no pencil or erasable ink). Accuracy Data should reflect the actual observation, measurement, or action without manipulation. Contemporaneous Recording...
by Dr. Yashashwini Reddy | Apr 19, 2025
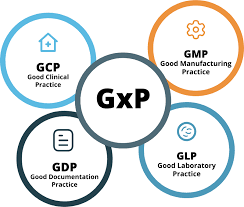
1. What are GLP, GDP, and GMP, and how do they differ? This foundational question tests your understanding of the definitions: GLP: Ensures integrity and quality of non-clinical lab studies. GDP: Ensures data integrity and accuracy in documentation. GMP: Ensures...





