
by Dr. Yashashwini Reddy | Sep 15, 2025
Self-Inspection and Quality Audits in Pharmaceuticals 1. Self-Inspection Definition: An internal examination carried out by the company itself to evaluate compliance with GMP, SOPs, and regulatory standards. Purpose: Detect deficiencies in the quality system. Verify...

by Dr. Yashashwini Reddy | Sep 15, 2025
Annual Product Quality Review (APQR/APR/PQR) in Quality Improvements 1. What is APQR/APR/PQR? Annual Product Quality Review (APQR), also known as Annual Product Review (APR) or Product Quality Review (PQR), is a regulatory requirement under ICH Q7, EU GMP Chapter 1...
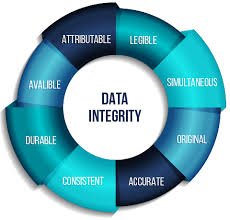
by Dr. Yashashwini Reddy | Sep 15, 2025
Audit Trail Requirements in Pharmaceuticals An audit trail is a secure, computer-generated, time-stamped record that allows for the reconstruction of events related to the creation, modification, or deletion of electronic data. In the pharmaceutical industry, audit...

by Dr. Yashashwini Reddy | Sep 15, 2025
FDA Forms Generally Used in Pharmaceutical Inspection 1. Form FDA 482 – Notice of Inspection Issued at the start of an inspection. Notifies the company that FDA investigators are officially beginning the inspection under the authority of the FD&C Act (Section...

by Dr. Yashashwini Reddy | Sep 15, 2025
Analytical Balances Drift and Its Importance 1. What is Analytical Balance Drift? Analytical balance drift refers to the gradual and unintentional change in the displayed weight reading of a balance over time, even without adding or removing material. This drift can...







