
by Dr. Yashashwini Reddy | May 8, 2025
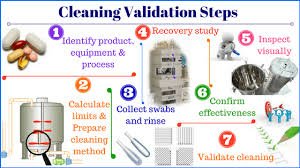
STANDARD OPERATING PROCEDURE (SOP) 1.0 OBJECTIVE To establish a standard procedure for cleaning the capsule filling line to ensure product quality, prevent cross-contamination, and comply with regulatory requirements. 2.0 SCOPE This SOP...
by Dr. Yashashwini Reddy | May 8, 2025
STANDARD OPERATING PROCEDURE (SOP) 1.0 OBJECTIVE To establish a standard procedure for the cleaning of equipment and accessories used in the production area to ensure cleanliness, prevent cross-contamination, and maintain product quality....

by Dr. Yashashwini Reddy | May 8, 2025
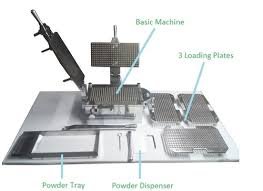
Standard Operating Procedure (SOP) 1.0 OBJECTIVE To lay down a procedure for cleaning the Deblistering Machine to ensure equipment cleanliness, avoid cross-contamination, and maintain GMP compliance. 2.0 SCOPE This procedure applies to all...
by Dr. Yashashwini Reddy | May 8, 2025
Standard Operating Procedure (SOP) This SOP ensures the cubicle remains clean, compliant with GMP standards, and ready for subsequent production batches. 1. Objective: To lay down the procedure for the proper cleaning of the capsule filling...

by Dr. Yashashwini Reddy | May 7, 2025
Standard Operating Procedure (SOP) 1. Objective To establish standardized procedures for reprocessing and reworking pharmaceutical products to ensure that all batches meet predefined specifications and maintain product quality. 2. Scope...







