
by Dr. Yashashwini Reddy | Aug 12, 2025
How to Submit a DMF (Drug Master File) A Drug Master File (DMF) is a confidential document submitted to the U.S. FDA that contains detailed information about the manufacturing, processing, packaging, and storage of drug substances, intermediates, or components.It is...

by Dr. Yashashwini Reddy | Aug 12, 2025
Understanding the US FDA Drug Approval Process The U.S. Food and Drug Administration (FDA) is responsible for ensuring that drugs marketed in the United States are safe, effective, and of high quality. The process is rigorous and involves multiple stages to verify...

by Dr. Yashashwini Reddy | Aug 12, 2025
How to Review GMP Documents Like a Pro 1. Understand the Purpose Identify what the document is for (e.g., SOP, batch record, protocol, report). Know the applicable GMP guidelines (US FDA, EMA, WHO, ICH). 2. Check for Compliance Ensure the document aligns with: Current...
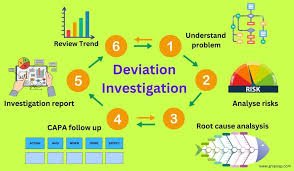
by Dr. Yashashwini Reddy | Aug 12, 2025
Deviation Control in Pharmaceuticals Definition:A deviation is any departure from approved instructions, standard operating procedures (SOPs), specifications, or established Good Manufacturing Practice (GMP) requirements during manufacturing, testing, packaging,...

by Dr. Yashashwini Reddy | Aug 11, 2025
Calibration of Automatic Potentiometric Titrator in Pharmaceuticals 1. Introduction An automatic potentiometric titrator is widely used in pharmaceutical laboratories for accurate and reproducible titrations, such as acid-base, redox, complexometric, and non-aqueous...







