by Dr. Yashashwini Reddy | Sep 23, 2025
📌 Case Study: COVID-19 Vaccine Development – Pfizer/BioNTech 🧩 Background In early 2020, the COVID-19 pandemic created an urgent global demand for a safe and effective vaccine. Pfizer (U.S.) partnered with BioNTech (Germany) to develop an mRNA-based vaccine (BNT162b2,...

by Dr. Yashashwini Reddy | Sep 23, 2025
📝 Case Study: OOS Investigation – Tablet Dissolution 📍 Background A marketed immediate-release tablet showed dissolution failure during routine quality control testing. Specification: NLT (Not Less Than) 80% drug release in 30 minutes. Observed result: 60–65% release...
by Dr. Yashashwini Reddy | Sep 23, 2025
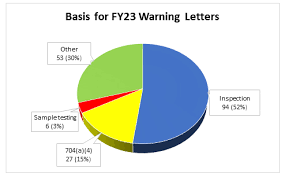
📌 Case Study: Data Integrity Failures – FDA Warning Letters 1. Background In recent years, several pharmaceutical companies (especially in India and China) have faced FDA Warning Letters and import alerts because of data integrity violations. These cases highlight the...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish a procedure for regular inspection of utilities (e.g., purified water, compressed air, HVAC system, steam, and gas supply) to ensure compliance with GMP requirements and operational efficiency. 2. Scope This procedure applies to all utility...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Premises and Equipment Use dedicated and clean facilities for production, storage, and filling of gases. Ensure gas cylinders, pipelines, valves, regulators, and manifolds are properly maintained and validated. Prevent cross-contamination between medical and...







