by Dr. Yashashwini Reddy | Aug 27, 2025
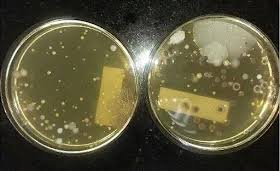
Elimination of Microbial Contamination from Classified Areas 1. Root Cause Identification Before elimination, identify why contamination occurred: Breach in personnel gowning or behavior. Ineffective cleaning/sanitization. HEPA filter leakage or improper airflow....
by Dr. Yashashwini Reddy | Aug 27, 2025
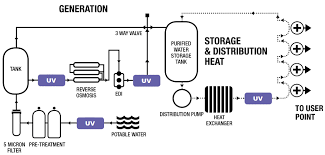
Risk Assessment for Purified Water System in Pharmaceuticals 1. Objective To identify, evaluate, and control risks associated with the design, operation, and maintenance of the Purified Water System (PWS), ensuring compliance with GMP, pharmacopeial standards, and...

by Dr. Yashashwini Reddy | Aug 27, 2025
Data Integrity in Microbial Analysis 1. Introduction In microbiology laboratories (especially in pharmaceutical QC/QA), data integrity ensures that all results of microbial testing are accurate, complete, consistent, and trustworthy throughout their lifecycle. Since...
by Dr. Yashashwini Reddy | Aug 27, 2025
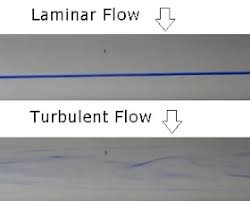
Reynolds Number and Its Significance in Purified Water System 1. What is Reynolds Number? Reynolds number (Re) is a dimensionless number that predicts the flow regime of a fluid (laminar, transitional, or turbulent). Formula: ...
by Dr. Yashashwini Reddy | Aug 27, 2025
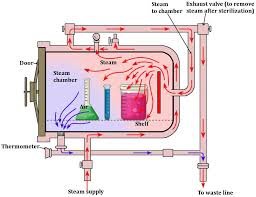
Principle and Working of Autoclave 1. Principle The autoclave works on the principle of moist heat sterilization using saturated steam under pressure. When pressure is applied to water, its boiling point increases. At 121 °C (15 psi / 1.05 kg/cm² above atmospheric...







