
by Dr. Yashashwini Reddy | Aug 29, 2025
1. Purpose To lay down the procedure for proper handling, operation, cleaning, and maintenance of microscopes to ensure accurate and reliable observations. 2. Scope This SOP is applicable to all microscopes (compound, stereo, digital, or phase-contrast) used in the...

by Dr. Yashashwini Reddy | Aug 28, 2025
1.0 Purpose To establish a standard procedure for the routine cleaning and disinfection of the sterile dress cabinet to ensure aseptic conditions are maintained for storage of sterile garments. 2.0 Scope This SOP applies to all sterile dress cabinets located in...

by Dr. Yashashwini Reddy | Aug 28, 2025
1.0 Purpose To define the procedure for proper handling, storage, usage, and disposal of biological indicators (BIs) used to monitor and validate sterilization processes (steam, dry heat, ethylene oxide, hydrogen peroxide, etc.). 2.0 Scope This SOP applies to all...

by Dr. Yashashwini Reddy | Aug 27, 2025
Fungus in Pharmaceutical Cleanrooms: Types, Origins, and Decontamination 1. Introduction Fungal contamination in cleanrooms is a major risk to sterile product manufacturing, since fungal spores are ubiquitous, resistant, and can survive in harsh conditions. Even low...
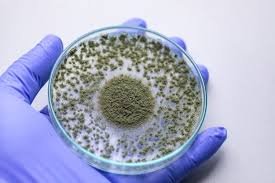
by Dr. Yashashwini Reddy | Aug 27, 2025
Fungus in Pharmaceutical Cleanrooms: Types, Origins, and Decontamination 1. Introduction Cleanrooms in pharmaceutical facilities are designed to maintain controlled levels of particles, microorganisms, and environmental parameters to ensure product sterility and...







