by Dr. Yashashwini Reddy | Aug 30, 2025
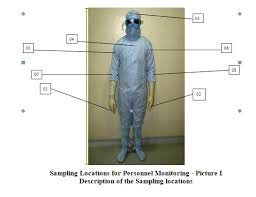
Standard Operating Procedure (SOP) for Microbiological Monitoring of Cleanroom 1. Purpose To describe the procedure for microbiological monitoring of cleanrooms to ensure controlled aseptic conditions and compliance with GMP guidelines. 2. Scope This SOP applies to...

by Dr. Yashashwini Reddy | Aug 29, 2025
1. Purpose To define the procedure for proper cleaning of the Dry Heat Sterilizer (DHS) to prevent contamination and ensure compliance with GMP requirements. 2. Scope This SOP is applicable to the cleaning of DHS used for depyrogenation/sterilization of vials,...

by Dr. Yashashwini Reddy | Aug 29, 2025
1. Purpose To describe the procedure for monitoring non-viable particulate matter in controlled manufacturing areas to ensure compliance with GMP, ISO 14644, and regulatory requirements. 2. Scope This SOP is applicable to all classified areas (Grade A, B, C, and D)...

by Dr. Yashashwini Reddy | Aug 29, 2025
1. Purpose To describe the procedure for monitoring microbiological contamination in controlled areas using the settle plate (passive air sampling) method. 2. Scope This SOP is applicable to all classified areas (Grade A/B/C/D) in manufacturing and microbiology...

by Dr. Yashashwini Reddy | Aug 29, 2025
1. Purpose To describe the procedure for performing microbiological integrity testing of sealed vials to ensure container closure integrity and sterility assurance. 2. Scope This SOP applies to all finished sterile product vials manufactured in the facility that...







