by Dr. Yashashwini Reddy | Sep 26, 2025
📌 Case Study: Sun Pharma / Caraco (US) – Recalls due to Contamination and CGMP Non-Compliance Background Caraco Pharmaceutical Laboratories, a US-based generic manufacturer and subsidiary of Sun Pharma, came under FDA scrutiny due to multiple quality issues. As one of...
by Dr. Yashashwini Reddy | Sep 26, 2025
Case Study: HeLa Cells & Informed Consent In 1951, Henrietta Lacks, an African-American woman, was treated for cervical cancer at Johns Hopkins Hospital. Without her knowledge or consent, doctors collected her tumor cells, which turned out to be the first human...
by Dr. Yashashwini Reddy | Sep 24, 2025
Case Study: Ranbaxy & Glass Particles Recall (2014) Background:In 2014, Ranbaxy Laboratories recalled several batches of injectable products in the United States due to the presence of glass particles in vials. The recall was classified as a Class II recall by the...

by Dr. Yashashwini Reddy | Sep 23, 2025
Case Study: Supply Chain Disruption – API Shortage Background A U.S.-based pharmaceutical company manufacturing essential cardiovascular and anti-diabetic medicines faced a sudden API (Active Pharmaceutical Ingredient) shortage in 2024. The primary API supplier was...
by Dr. Yashashwini Reddy | Sep 23, 2025
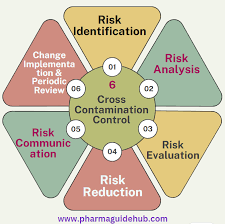
🧪 Case Study: Cross-Contamination in a Multi-Product Pharmaceutical Facility 📌 Background A European pharmaceutical manufacturer operated a multi-product solid oral dosage plant. During a routine EMA inspection, regulatory authorities found traces of a potent API...







