
by Dr. Yashashwini Reddy | Sep 6, 2025
How to Review GMP Documents Like a Pro 1. Understand the Purpose of the Document Identify the type: SOP, Batch Manufacturing Record (BMR), Protocol, Validation Report, Logbook, etc. Ask: What is this document supposed to control, guide, or prove? 2. Verify Structure...

by Dr. Yashashwini Reddy | Sep 6, 2025
1.0 Purpose To lay down the procedure for the safe, secure, and documented transfer of finished goods from the production/packing area to the bonded store room under controlled conditions to ensure compliance with regulatory and company requirements. 2.0 Scope This...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To describe the procedure for operation, cleaning, and maintenance of the Cad Mill to ensure consistent particle size reduction and compliance with cGMP requirements. 2.0 Scope This SOP applies to the Cad Mill installed in the production area of [Company...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To establish a uniform procedure for the operation, cleaning, and maintenance of the conveyor belt used in the packing section to ensure smooth functioning and compliance with cGMP requirements. 2.0 Scope This SOP applies to all conveyor belts used in the...
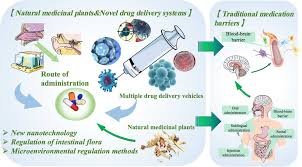
by Dr. Yashashwini Reddy | Sep 3, 2025
Novel Drug Delivery Systems (NDDS) A Novel Drug Delivery System is a modern approach of formulating and delivering drugs to improve efficacy, safety, patient compliance, and targeted delivery compared to conventional dosage forms (like tablets, capsules, or...







