
by Dr. Yashashwini Reddy | Sep 6, 2025
Pharmaceutical companies operate under strict cGMP regulations enforced by agencies like FDA, EMA, MHRA, and WHO. Even well-established firms make compliance mistakes that can lead to FDA 483s, Warning Letters, recalls, or import alerts. Below are 8 of the most common...

by Dr. Yashashwini Reddy | Sep 6, 2025
Data Falsification in the Pharmaceutical Industry 1. What is Data Falsification? Data falsification in pharmaceuticals refers to the intentional manipulation, fabrication, or misrepresentation of data in order to conceal deviations, meet specifications, or mislead...
by Dr. Yashashwini Reddy | Sep 6, 2025
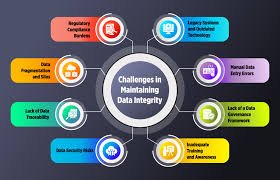
Importance of Data Integrity for Pharmaceutical Regulatory Agencies 1. What is Data Integrity? Data integrity means maintaining the accuracy, completeness, consistency, and reliability of data throughout its lifecycle, ensuring it is attributable, legible,...

by Dr. Yashashwini Reddy | Sep 6, 2025
The Importance of FDA Form 483s and Warning Letters in Pharmaceuticals 1. What is FDA Form 483? FDA Form 483, “Inspectional Observations,” is issued by FDA inspectors to company management at the end of an inspection. It lists potential violations of the Food, Drug,...
by Dr. Yashashwini Reddy | Sep 6, 2025
Regulatory Expectations from Cleaning Validation Cleaning validation is a documented evidence-based process to demonstrate that cleaning procedures effectively and consistently remove residues of products, cleaning agents, and contaminants to predetermined acceptable...







