
by Dr. Yashashwini Reddy | Sep 6, 2025
Case Studies: Troubleshooting Purified Water System Failures A Purified Water (PW) System must consistently supply water that meets pharmacopeial standards (USP, EP, IP, JP) for use in manufacturing, cleaning, and testing. Failures in the system can lead to OOS...
by Dr. Yashashwini Reddy | Sep 6, 2025
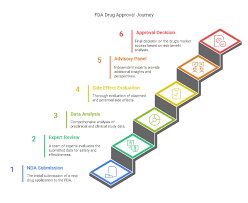
5 Steps of FDA Approvals The U.S. Food and Drug Administration (FDA) follows a structured process to ensure that drugs are safe, effective, and high-quality before they reach patients. 1. Preclinical Testing (Laboratory & Animal Studies) Conducted before human...

by Dr. Yashashwini Reddy | Sep 6, 2025
Requirements of FDA for Training in Pharmaceuticals Training in the pharmaceutical industry is a critical requirement under cGMP regulations (21 CFR Parts 210 & 211). The FDA expects companies to have a structured, documented training system that ensures employees...
by Dr. Yashashwini Reddy | Sep 6, 2025
Planning and Procedure Followed During Regulatory Audits Regulatory audits (by USFDA, EMA, MHRA, WHO, or local authorities) are conducted to verify compliance with cGMP and regulatory standards. Effective preparation and systematic execution are crucial for success....

by Dr. Yashashwini Reddy | Sep 6, 2025
10 Step Guide to cGMP Certification cGMP (Current Good Manufacturing Practices) certification demonstrates that a company’s facilities, processes, and quality systems comply with international regulatory standards (FDA, EMA, WHO, PIC/S). It assures product quality,...






