by Dr. Yashashwini Reddy | Sep 9, 2025
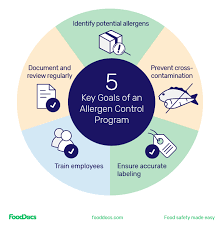
🧾 Allergen Control Plan for Pharmaceuticals 1. Risk Assessment Identify allergenic excipients (e.g., lactose, soy lecithin, egg-derived albumin, peanut oil, gluten, gelatin). Assess risk of cross-contamination during manufacturing, packaging, storage, and cleaning....
by Dr. Yashashwini Reddy | Sep 9, 2025
✅ Areas to Inspect During Self-Inspection in Pharmaceuticals Personnel & Training Verify staff qualifications, training records, and adherence to GMP practices. Premises & Facilities Check cleanliness, maintenance, pest control, HVAC systems, and environmental...

by Dr. Yashashwini Reddy | Sep 9, 2025
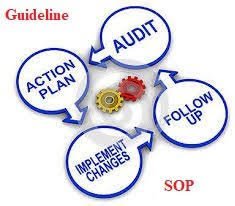
🔑 Tips for Effective Internal Audit System Establish Clear Objectives Define the purpose of the audit (compliance, efficiency, risk identification, or process improvement). Develop a Comprehensive Audit Plan Prepare a risk-based annual audit schedule covering all...
by Dr. Yashashwini Reddy | Sep 8, 2025
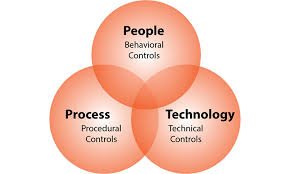
Impact of Culture and Behaviors on Data Integrity Compliance in Pharmaceuticals Data integrity is not only about systems and SOPs, but also about people’s mindset, culture, and daily behaviors. Even the best electronic systems and QMS can fail if employees lack the...

by Dr. Yashashwini Reddy | Sep 8, 2025
Recent GMP Violations at Indian Pharma Facilities 1. Granules India (Telangana) In a 2024 inspection, the FDA observed severe cross-contamination issues: residues in air ducts, microbial contamination despite HEPA filters, bird droppings and feathers in production...







