
by Dr. Yashashwini Reddy | Sep 10, 2025
✅ 4 Tips to Reduce 483 Observations 1. Strengthen Documentation Practices Ensure records are contemporaneous, complete, and accurate (ALCOA+ principles). Use controlled logbooks, proper version control, and audit trails. Train staff to document activities at the time...

by Dr. Yashashwini Reddy | Sep 10, 2025
🔎 Major Audit Findings – Equipment & Instruments 1. Qualification & Validation Issues Missing or incomplete DQ/IQ/OQ/PQ records. Equipment used without initial or periodic requalification. Validation protocols not followed or not approved before execution....
by Dr. Yashashwini Reddy | Sep 10, 2025
🔴 Critical Mistakes in Root Cause Investigation (RCI): Jumping to Conclusions – Assuming the cause without evidence or proper investigation. Superficial Investigation – Stopping at symptoms instead of identifying the true underlying cause. Poor Documentation –...
by Dr. Yashashwini Reddy | Sep 9, 2025
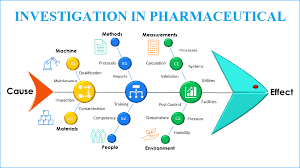
🐟 Fishbone Tool of Investigation in Pharmaceuticals 📌 What is It? A cause-and-effect diagram shaped like a fish skeleton. “Head” = problem statement (e.g., OOS result, contamination, deviation). “Bones” = major categories of potential causes. Helps investigation teams...

by Dr. Yashashwini Reddy | Sep 9, 2025
⚠️ 5 Most Common FDA 483 Observations in Pharma 1. Inadequate Investigations (OOS / Deviations / Complaints) Failure to thoroughly investigate out-of-specification (OOS) results, deviations, or complaints. Root cause analysis either incomplete, unjustified, or not...







