
by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 GMP Audit Checklist – Storage of Starting Materials 1. Material Receipt & Identification ✅ Are incoming starting materials received against approved suppliers and purchase orders? ✅ Are materials inspected for damage, tampering, contamination, and labeling...
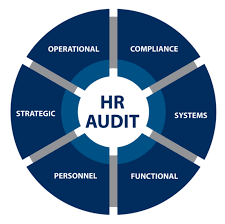
by Dr. Yashashwini Reddy | Sep 10, 2025
👥 Checklist for Audit in HR / Admin (Pharmaceuticals) 1. Organization & Policies ✅ Organization chart updated and approved. ✅ HR policies documented (recruitment, promotion, disciplinary action). ✅ Job descriptions (JDs) defined, approved, and role-specific. ✅...

by Dr. Yashashwini Reddy | Sep 10, 2025
📦 Checklist for Audit in Warehouse (Pharmaceuticals) 1. General Warehouse Conditions ✅ Clean, well-organized, and free from dust, pests, and waste. ✅ Adequate space for material movement and segregation. ✅ Environmental monitoring records available (temperature,...

by Dr. Yashashwini Reddy | Sep 10, 2025
⚖️ Analytical Balance Drift 🔎 What is Drift? Drift refers to the gradual change in the displayed weight reading of an analytical balance over time without any actual change in the sample’s mass. It is typically observed when the balance reading keeps increasing or...

by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Preparation for GMP Audit in Pharmaceuticals 1. Understand the Audit Scope Know whether it’s regulatory (FDA, EMA, MHRA, WHO, CDSCO), customer, or internal. Review previous audit/inspection reports and ensure CAPAs are implemented. Be aware of current guidelines,...







