
by Dr. Yashashwini Reddy | Oct 9, 2025
Elaboration:The development and manufacture of drug substances (Active Pharmaceutical Ingredients – API) encompass all stages from the initial synthesis or isolation of the active compound to its large-scale commercial production under Good Manufacturing Practice...
by Dr. Yashashwini Reddy | Oct 9, 2025
A Pharmaceutical Quality System (PQS) is a comprehensive framework that ensures the consistent quality, safety, and efficacy of pharmaceutical products throughout their life cycle — from development to discontinuation. It aligns with ICH Q10, which complements ICH Q8...
by Dr. Yashashwini Reddy | Oct 9, 2025
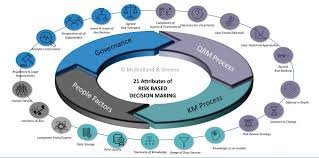
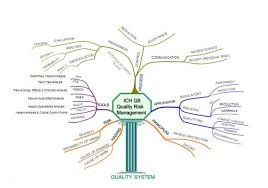
Quality Risk Management (QRM) is a systematic process for the assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. The purpose of QRM is to ensure that product quality, patient safety, and regulatory...

by Dr. Yashashwini Reddy | Oct 8, 2025
🧪 ICH Q2: Analytical Validation 🔹 Full Title: ICH Q2(R2) – Validation of Analytical Procedures 🔹 Objective: To provide guidance on the validation of analytical methods used to test drug substances and products — ensuring that the methods are accurate, reliable, and...

by Dr. Yashashwini Reddy | Oct 8, 2025
🧭 Quality Guidelines – Overview Quality Guidelines are internationally harmonized standards developed mainly by the International Council for Harmonisation (ICH) and other regulatory authorities (like FDA, EMA, WHO, CDSCO).They help ensure that pharmaceutical products...







