by Dr. Yashashwini Reddy | Oct 9, 2025
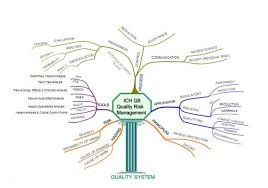
Quality Risk Management (QRM) is a systematic process for the assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. The purpose of QRM is to ensure that product quality, patient safety, and regulatory...
by Dr. Yashashwini Reddy | Aug 9, 2025
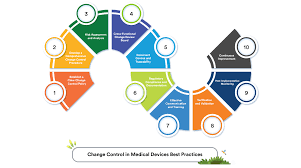
4 Steps to Effective Change Control in Pharmaceuticals 1. Initiation & Documentation Identify the need for change (equipment, process, material, method, etc.). Document the proposed change in a Change Control Form with details like scope, reason, impact area, and...





