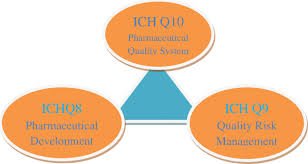
by Dr. Yashashwini Reddy | Oct 9, 2025
Pharmaceutical development, as described in ICH Q8 (R2), is the process of designing a quality product and its manufacturing process to consistently deliver the intended performance. The goal is to understand how formulation and process variables influence product...

by Dr. Yashashwini Reddy | Aug 8, 2025
Strategies for Resolving Stability Issues in Drug Formulations Stability issues can arise due to chemical degradation, physical changes, or microbial contamination, leading to reduced efficacy, altered safety, or shortened shelf life. The resolution process involves...

by Dr. Yashashwini Reddy | May 2, 2025
Preparation of Master Formula Record (MFR) in Pharmaceutical Manufacturing The Master Formula Record (MFR) is a critical document in pharmaceutical manufacturing. It serves as a detailed guide for producing a specific batch of a pharmaceutical product, ensuring that...

by Dr. Yashashwini Reddy | Apr 28, 2025
Enhancing the stability of pharmaceutical formulations is a critical aspect of drug development and manufacturing. Stability ensures that the active ingredients in a drug maintain their potency, safety, and effectiveness throughout the product’s shelf life....
by Dr. Yashashwini Reddy | Oct 5, 2024
Exploring the HLB Scale in Pharmaceuticals: Significance and Applications The Hydrophilic-Lipophilic Balance (HLB) system serves as an essential tool in pharmaceutical formulations, aiding in the selection of surfactants and emulsifiers for products such as lotions,...






