by Dr. Yashashwini Reddy | Sep 23, 2025
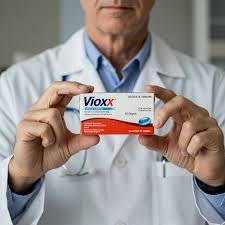
Case Study: Pharmacovigilance Signal Detection – Vioxx (Rofecoxib) Background Drug: Rofecoxib (Brand name: Vioxx) Company: Merck & Co. Indication: Relief of pain and inflammation in osteoarthritis, rheumatoid arthritis, and acute pain. Launch: 1999 Mechanism:...

by Dr. Yashashwini Reddy | Sep 11, 2025
📌 EMA vs FDA Expectations on Process Validation Definition:Process Validation (PV) is the collection and evaluation of data, from process design to commercial production, to establish scientific evidence that a process is capable of consistently delivering quality...

by Dr. Yashashwini Reddy | Aug 18, 2025
FDA Requirements for Training in Pharmaceuticals The U.S. Food and Drug Administration (FDA), under 21 CFR Part 211 (cGMP for Finished Pharmaceuticals) and ICH Q10 Pharmaceutical Quality System, outlines clear expectations for employee training. 1. Training Program...
by Dr. Yashashwini Reddy | Jun 4, 2024
Drug Master File Submission Process We have discussed the types of drug master files and their purpose in the previous article now we will learn how a DMF is submitted to the FDA in short. Procedure for DMF submission Electronic Common Technical Document (eCTD): DMFs...
by Dr. Yashashwini Reddy | Jun 4, 2024
In this article, you are going to learn about the regulatory procedure for combination products in the US. What is RFD what are the timelines for it and what role of the Office of Combination products? Combination products as discussed in the article are medicinal...





