by Dr. Yashashwini Reddy | Sep 20, 2025
1. Objective To ensure that equipment, system, or instrument is installed correctly at the user site and performs as per the predefined specifications, operational requirements, and approved protocols. 2. Scope Applicable to all new equipment, utilities, systems, and...

by Dr. Yashashwini Reddy | Sep 15, 2025
Major Audit Findings about Equipment and Instruments in Pharmaceuticals Equipment and instruments are critical for ensuring product quality, reliability of test results, and compliance with cGMP. Regulatory inspections (FDA, EMA, MHRA, WHO) often highlight...

by Dr. Yashashwini Reddy | Aug 18, 2025
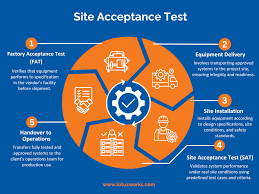
Site Acceptance Test (SAT) The Site Acceptance Test (SAT) is the final phase of equipment/system qualification, conducted at the customer’s site after installation and commissioning. It verifies that the system, machine, or equipment functions correctly in the actual...

by Dr. Yashashwini Reddy | Aug 12, 2025
How to Write a Validation Protocol A Validation Protocol is a formal, approved document describing how validation will be performed, including the methodology, acceptance criteria, responsibilities, and documentation requirements.It applies to Process Validation,...

by Dr. Yashashwini Reddy | Aug 8, 2025
User Requirement Specification (URS) of Equipment 1. Definition The URS is a document prepared by the end user that clearly defines the functional, operational, and regulatory requirements an equipment must meet before procurement or qualification.It serves as a...







