
by Dr. Yashashwini Reddy | Sep 15, 2025
📘 Guidelines for Preparation of Site Master File (SMF) The Site Master File (SMF) is a regulatory document that provides detailed information about a pharmaceutical manufacturing site, operations, and quality management system. It is required by WHO, EU GMP (Annex...
by Dr. Yashashwini Reddy | Sep 15, 2025
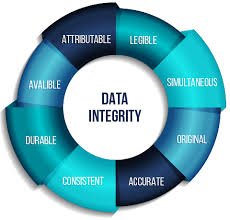
Audit Trail Requirements in Pharmaceuticals An audit trail is a secure, computer-generated, time-stamped record that allows for the reconstruction of events related to the creation, modification, or deletion of electronic data. In the pharmaceutical industry, audit...
by Dr. Yashashwini Reddy | Sep 13, 2025
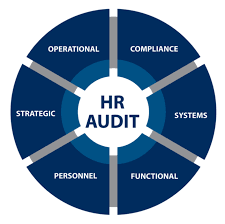
HR Audit Checklist 1. Recruitment & Selection Existence of recruitment policies and SOPs. Proper manpower requisition approvals before hiring. Background verification and reference checks of employees. Job descriptions and competency requirements documented....

by Dr. Yashashwini Reddy | Sep 8, 2025
7 Steps for Monitoring Compliance in Pharmaceuticals 1. Establish a Robust Quality Management System (QMS) Build SOPs, policies, and guidelines aligned with FDA, EMA, and ICH standards. Ensure document control and version management. 2. Conduct Regular Internal Audits...

by Dr. Yashashwini Reddy | Sep 6, 2025
Case Studies: Troubleshooting Purified Water System Failures A Purified Water (PW) System must consistently supply water that meets pharmacopeial standards (USP, EP, IP, JP) for use in manufacturing, cleaning, and testing. Failures in the system can lead to OOS...







