
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish a procedure for regular inspection of utilities (e.g., purified water, compressed air, HVAC system, steam, and gas supply) to ensure compliance with GMP requirements and operational efficiency. 2. Scope This procedure applies to all utility...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Premises and Equipment Use dedicated and clean facilities for production, storage, and filling of gases. Ensure gas cylinders, pipelines, valves, regulators, and manifolds are properly maintained and validated. Prevent cross-contamination between medical and...
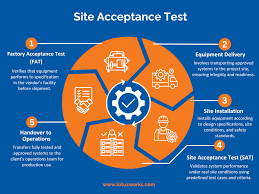
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Objective To ensure that equipment, system, or instrument is installed correctly at the user site and performs as per the predefined specifications, operational requirements, and approved protocols. 2. Scope Applicable to all new equipment, utilities, systems, and...

by Dr. Yashashwini Reddy | Sep 18, 2025
📌 Lux (Light Intensity) Standards in Pharma Industry Light intensity in pharmaceutical facilities is crucial for ensuring product quality, safety, and compliance with cGMP and cleanroom requirements. It is measured in lux (lx), which represents lumens per square...

by Dr. Yashashwini Reddy | Sep 16, 2025
1.0 Purpose To describe the procedure for proper cleaning, maintenance, and storage of sampling equipment used for raw materials, intermediates, and finished products to avoid contamination, cross-contamination, and ensure equipment integrity. 2.0 Scope This SOP...







