
by Dr. Yashashwini Reddy | Aug 29, 2025
1. Purpose To describe the procedure for monitoring microbiological contamination in controlled areas using the settle plate (passive air sampling) method. 2. Scope This SOP is applicable to all classified areas (Grade A/B/C/D) in manufacturing and microbiology...

by Dr. Yashashwini Reddy | Aug 29, 2025
1. Purpose To define the procedure for calibration of the Slit-to-Agar Air Sampler used for environmental monitoring to ensure accurate measurement of microbial air contamination. 2. Scope This SOP applies to all STA air samplers used in microbiology or cleanroom...
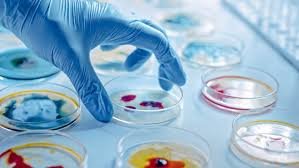
by Dr. Yashashwini Reddy | Aug 27, 2025
Best Practices in Pharmaceutical Microbiology Laboratory Facility & Environment Maintain controlled cleanroom environments with appropriate classification (ISO/GMP requirements). Ensure unidirectional personnel and material flow to minimize contamination risk. Use...

by Dr. Yashashwini Reddy | Aug 18, 2025
Contamination Control Strategies for Manufacturing Area In pharmaceutical manufacturing, contamination control is critical to ensure product quality, patient safety, and regulatory compliance. Contamination can occur in the form of particulate, microbial, chemical, or...

by Dr. Yashashwini Reddy | May 2, 2025
The validation of clean room pass boxes is a critical process in pharmaceutical manufacturing, ensuring that these systems effectively maintain a sterile or controlled environment when transferring materials or products between different areas (e.g., from non-sterile...







