by Dr. Yashashwini Reddy | Oct 8, 2025
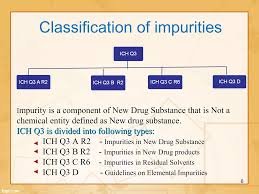
Elaboration:Impurities are unwanted chemicals that remain with the active pharmaceutical ingredient (API) or develop during formulation and storage. According to ICH Q3A (for new drug substances) and ICH Q3B (for new drug products), impurities must be identified,...
by Dr. Yashashwini Reddy | Nov 27, 2024
Interview Questions and Answers for Drug Substance (3.2.S) in CTD – Regulatory Affairs (Freshers) 1. What is the role of 3.2.S in the CTD (Common Technical Document)? Answer: The 3.2.S section of the CTD is dedicated to the “Drug Substance,” which...
by Dr. Yashashwini Reddy | Jun 4, 2024
Drug Master File Submission Process We have discussed the types of drug master files and their purpose in the previous article now we will learn how a DMF is submitted to the FDA in short. Procedure for DMF submission Electronic Common Technical Document (eCTD): DMFs...



