by Dr. Yashashwini Reddy | Oct 8, 2025
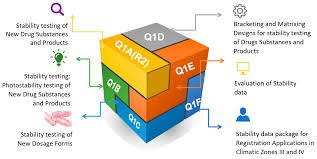
📘 ICH Q1: Stability Testing 🔹 Purpose The ICH Q1 series provides guidance on how to design stability studies to ensure that drug substances and drug products maintain their quality, safety, and efficacy throughout their shelf life under various environmental...

by Dr. Yashashwini Reddy | Aug 8, 2025
Strategies for Resolving Stability Issues in Drug Formulations Stability issues can arise due to chemical degradation, physical changes, or microbial contamination, leading to reduced efficacy, altered safety, or shortened shelf life. The resolution process involves...

by Dr. Yashashwini Reddy | Aug 8, 2025
Understanding the stability of injectable products is crucial because these formulations are typically sterile, sensitive to environmental factors, and often contain active pharmaceutical ingredients (APIs) that can degrade quickly if not stored or handled properly....

by Dr. Yashashwini Reddy | Apr 19, 2025
1. What are stability studies and why are they important? Stability studies determine how the quality of a drug substance or product varies over time under the influence of environmental factors (e.g., temperature, humidity, light). They are essential for setting...
by Dr. Yashashwini Reddy | Nov 25, 2024
Forced Degradation Studies in Pharmaceuticals Introduction Forced degradation studies are essential in the pharmaceutical industry for evaluating the stability of drug substances and formulations. These studies involve exposing drugs to extreme environmental...






