
by Dr. Yashashwini Reddy | Sep 8, 2025
7 Steps for Monitoring Compliance in Pharmaceuticals 1. Establish a Robust Quality Management System (QMS) Build SOPs, policies, and guidelines aligned with FDA, EMA, and ICH standards. Ensure document control and version management. 2. Conduct Regular Internal Audits...

by Dr. Yashashwini Reddy | Sep 8, 2025
Drug recalls can harm patients, damage reputation, and lead to regulatory penalties. To minimize risks, pharmaceutical companies should adopt a proactive quality and compliance strategy. 1. Strong Quality Management System (QMS) Implement robust SOPs across...

by Dr. Yashashwini Reddy | Sep 6, 2025
Case Studies: Troubleshooting Purified Water System Failures A Purified Water (PW) System must consistently supply water that meets pharmacopeial standards (USP, EP, IP, JP) for use in manufacturing, cleaning, and testing. Failures in the system can lead to OOS...
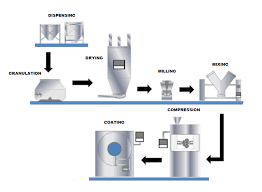
by Dr. Yashashwini Reddy | Sep 2, 2025
🔹 Tablet Manufacturing Process: An Overview Tablets are solid dosage forms containing one or more active pharmaceutical ingredients (APIs) with suitable excipients. The manufacturing process must ensure uniformity, stability, safety, and efficacy. 1. Pre-Formulation...

by Dr. Yashashwini Reddy | Sep 2, 2025
🔹 1. Process Optimization Lean Manufacturing (Lean Pharma): Eliminate non-value-added steps, reduce waiting times, optimize material flow. Six Sigma & QbD (Quality by Design): Use statistical tools (DoE, risk assessment) to identify Critical Process Parameters...







