by Dr. Yashashwini Reddy | Sep 10, 2025
🔴 Critical Mistakes in Root Cause Investigation (RCI): Jumping to Conclusions – Assuming the cause without evidence or proper investigation. Superficial Investigation – Stopping at symptoms instead of identifying the true underlying cause. Poor Documentation –...
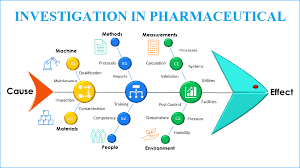
by Dr. Yashashwini Reddy | Sep 9, 2025
🐟 Fishbone Tool of Investigation in Pharmaceuticals 📌 What is It? A cause-and-effect diagram shaped like a fish skeleton. “Head” = problem statement (e.g., OOS result, contamination, deviation). “Bones” = major categories of potential causes. Helps investigation teams...

by Dr. Yashashwini Reddy | Sep 9, 2025
🧾 Common FDA 483 Observations Related to Cleaning in Pharmaceuticals 1. Inadequate Cleaning Validation Cleaning validation not performed for all product-contact equipment. Worst-case product selection (hardest to clean, most toxic/potent, least soluble) not justified....
by Dr. Yashashwini Reddy | Sep 8, 2025
Impact of Culture and Behaviors on Data Integrity Compliance in Pharmaceuticals Data integrity is not only about systems and SOPs, but also about people’s mindset, culture, and daily behaviors. Even the best electronic systems and QMS can fail if employees lack the...
by Dr. Yashashwini Reddy | Sep 8, 2025
Planning and Execution of Internal Audits in Pharmaceuticals Internal audits (self-inspections) are a key part of a pharmaceutical Quality Management System (QMS). They ensure compliance with cGMP, regulatory guidelines, and company SOPs, while also driving continuous...






