by Dr. Yashashwini Reddy | Sep 26, 2025
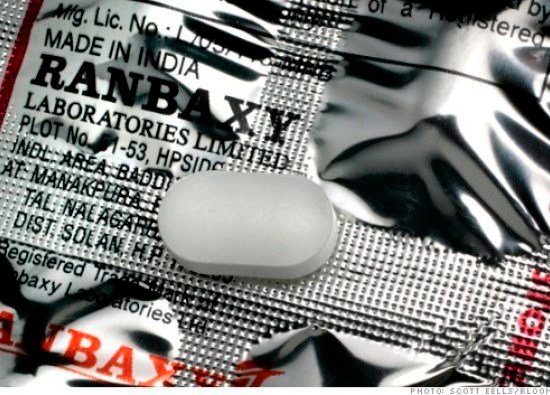
📌 Case Study: Ranbaxy Laboratories – Data Falsification & GMP Violations Background:Ranbaxy Laboratories, once India’s largest generic drug manufacturer, faced one of the biggest regulatory scandals in the pharmaceutical industry. In 2013, the company admitted to...
by Dr. Yashashwini Reddy | Sep 24, 2025
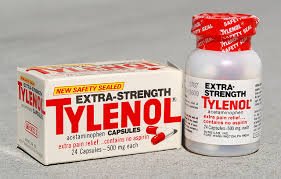
Case Study: Tylenol Cyanide Crisis (1982) Background Johnson & Johnson’s Tylenol was a leading over-the-counter (OTC) pain reliever in the U.S. In 1982, an incident shocked the pharmaceutical industry and the public health system, raising questions about drug...
by Dr. Yashashwini Reddy | Sep 23, 2025
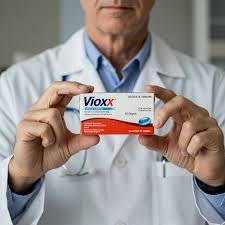
Case Study: Pharmacovigilance Signal Detection – Vioxx (Rofecoxib) Background Drug: Rofecoxib (Brand name: Vioxx) Company: Merck & Co. Indication: Relief of pain and inflammation in osteoarthritis, rheumatoid arthritis, and acute pain. Launch: 1999 Mechanism:...

by Dr. Yashashwini Reddy | Sep 18, 2025
📌 Importance of Differential Pressure in Pharmaceuticals Prevents Cross-Contamination Differential pressure ensures controlled airflow between cleanrooms of different classifications. Positive pressure in cleaner areas prevents entry of dust, microbes, and...

by Dr. Yashashwini Reddy | Sep 16, 2025
1.0 Purpose To describe the procedure for proper cleaning, maintenance, and storage of sampling equipment used for raw materials, intermediates, and finished products to avoid contamination, cross-contamination, and ensure equipment integrity. 2.0 Scope This SOP...







