by Dr. Yashashwini Reddy | Oct 9, 2025
Pharmaceutical development, as described in ICH Q8 (R2), is the process of designing a quality product and its manufacturing process to consistently deliver the intended performance. The goal is to understand how formulation and process variables influence product...
by Dr. Yashashwini Reddy | Oct 8, 2025
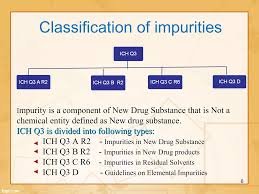
Elaboration:Impurities are unwanted chemicals that remain with the active pharmaceutical ingredient (API) or develop during formulation and storage. According to ICH Q3A (for new drug substances) and ICH Q3B (for new drug products), impurities must be identified,...
by Dr. Yashashwini Reddy | Nov 27, 2024
Interview Questions and Answers on Module 3.2.P (Drug Product) Under Quality – CTD 1. What is the 3.2.P section of the CTD? Answer: The 3.2.P section in Module 3 of the CTD deals with the drug product. It provides detailed information about the finished dosage...
by Dr. Yashashwini Reddy | Jun 4, 2024
Drug Master File Submission Process We have discussed the types of drug master files and their purpose in the previous article now we will learn how a DMF is submitted to the FDA in short. Procedure for DMF submission Electronic Common Technical Document (eCTD): DMFs...





