by Dr. Yashashwini Reddy | Sep 20, 2025
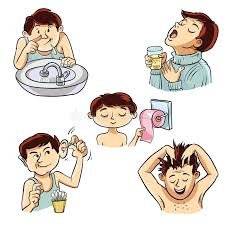
1. Purpose To establish guidelines for maintaining personal hygiene of all personnel to ensure product safety, prevent contamination, and maintain a clean working environment. 2. Scope This SOP applies to all employees, contract workers, visitors, and service...
by Dr. Yashashwini Reddy | Sep 20, 2025
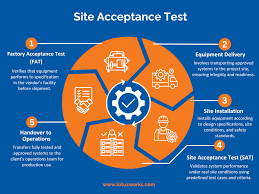
1. Objective To ensure that equipment, system, or instrument is installed correctly at the user site and performs as per the predefined specifications, operational requirements, and approved protocols. 2. Scope Applicable to all new equipment, utilities, systems, and...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To verify and document that the equipment/system performs consistently and reliably under actual production conditions as per predefined specifications and acceptance criteria. 2. Scope This PQ is applicable to [Equipment/System Name] used in...
by Dr. Yashashwini Reddy | Sep 18, 2025
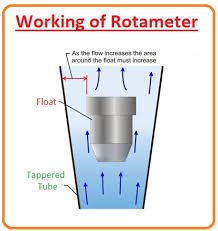
📌 Rotameter A Rotameter is a simple, reliable device used to measure the flow rate of liquids and gases in pharmaceutical, chemical, and other industries. It belongs to the variable area flowmeter family. 🔬 Principle of Rotameter Works on the variable area principle:...

by Dr. Yashashwini Reddy | Sep 18, 2025
📌 Importance of Differential Pressure in Pharmaceuticals Prevents Cross-Contamination Differential pressure ensures controlled airflow between cleanrooms of different classifications. Positive pressure in cleaner areas prevents entry of dust, microbes, and...







