by Dr. Yashashwini Reddy | Aug 18, 2025
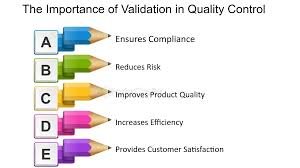
Importance of Validation in Pharmaceuticals Validation in pharmaceuticals is a critical quality assurance tool that ensures products are consistently manufactured to meet predetermined specifications and regulatory requirements. Its importance can be summarized as...

by Dr. Yashashwini Reddy | Aug 18, 2025
Recovery Factor Determination in Cleaning Validation Definition:Recovery Factor (RF) is the percentage of analyte recovered from a surface during cleaning validation studies. It helps to account for possible losses during swabbing/rinsing and analytical testing,...

by Dr. Yashashwini Reddy | Aug 18, 2025
Three Consecutive Batches for Validation in Pharmaceuticals In the pharmaceutical industry, process validation is a critical requirement to demonstrate that a manufacturing process consistently produces a product meeting predetermined quality attributes. Why three...
by Dr. Yashashwini Reddy | Aug 12, 2025
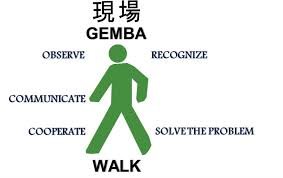
Gemba Walks and Their Implementation in Pharmaceuticals 1. What is a Gemba Walk? Gemba is a Japanese term meaning “the real place”—where work actually happens. In lean manufacturing and quality management, a Gemba Walk is when leaders, managers, or QA personnel go to...

by Dr. Yashashwini Reddy | Aug 12, 2025
How to Review GMP Documents Like a Pro 1. Understand the Purpose Identify what the document is for (e.g., SOP, batch record, protocol, report). Know the applicable GMP guidelines (US FDA, EMA, WHO, ICH). 2. Check for Compliance Ensure the document aligns with: Current...







