by Dr. Yashashwini Reddy | Sep 2, 2025
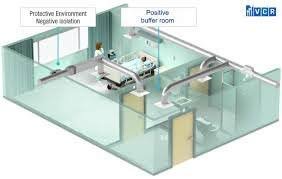
🔹 Buffer Area in Sterile Facility 1. Definition The Buffer Area (also called the cleanroom or critical area) is a Class 100 / ISO 5 / Grade A-B environment where aseptic processing, sterile filling, and direct product exposure operations are performed. It is...
by Dr. Yashashwini Reddy | Sep 2, 2025
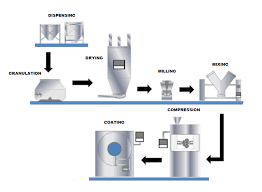
🔹 Tablet Manufacturing Process: An Overview Tablets are solid dosage forms containing one or more active pharmaceutical ingredients (APIs) with suitable excipients. The manufacturing process must ensure uniformity, stability, safety, and efficacy. 1. Pre-Formulation...

by Dr. Yashashwini Reddy | Sep 2, 2025
🔹 1. Process Optimization Lean Manufacturing (Lean Pharma): Eliminate non-value-added steps, reduce waiting times, optimize material flow. Six Sigma & QbD (Quality by Design): Use statistical tools (DoE, risk assessment) to identify Critical Process Parameters...

by Dr. Yashashwini Reddy | Sep 2, 2025
1. Influence on Stability Moisture Sensitivity: Smaller granules have a larger surface area, which can increase moisture absorption, leading to hydrolytic degradation of moisture-sensitive drugs. Oxidation: Increased surface area also accelerates oxidative...

by Dr. Yashashwini Reddy | Sep 2, 2025
Generic Drugs Manufacturing: Opportunities and Obstacles Opportunities: Cost-effectiveness: Generic drugs offer patients affordable alternatives to branded medicines, increasing accessibility and market demand. Patent Expiry of Blockbuster Drugs: As patents of many...







