
by Dr. Yashashwini Reddy | Sep 8, 2025
Difference Between FDA Form 483 and Warning Letter 1. FDA Form 483 (Inspectional Observations) Issued when: FDA inspectors observe potential violations of the Food, Drug, and Cosmetic Act (FD&C Act) during an inspection. Purpose: To notify company management of...

by Dr. Yashashwini Reddy | Sep 8, 2025
7 Steps for Monitoring Compliance in Pharmaceuticals 1. Establish a Robust Quality Management System (QMS) Build SOPs, policies, and guidelines aligned with FDA, EMA, and ICH standards. Ensure document control and version management. 2. Conduct Regular Internal Audits...

by Dr. Yashashwini Reddy | Sep 8, 2025
1. Be Inspection-Ready Always Keep documentation, equipment, and facilities in a state of compliance at all times. Conduct regular self-inspections and mock audits. Ensure all records are updated, accurate, and readily retrievable. 2. Train Employees on GMP and...
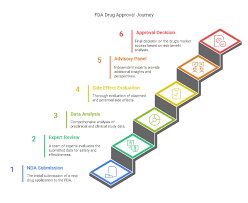
by Dr. Yashashwini Reddy | Sep 6, 2025
5 Steps of FDA Approvals The U.S. Food and Drug Administration (FDA) follows a structured process to ensure that drugs are safe, effective, and high-quality before they reach patients. 1. Preclinical Testing (Laboratory & Animal Studies) Conducted before human...

by Dr. Yashashwini Reddy | Sep 6, 2025
Pharmaceutical companies operate under strict cGMP regulations enforced by agencies like FDA, EMA, MHRA, and WHO. Even well-established firms make compliance mistakes that can lead to FDA 483s, Warning Letters, recalls, or import alerts. Below are 8 of the most common...







