
by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Guidelines for Preparation of Site Master File (SMF) 📑 1. General Information Company name, address, and contact details. Name of parent company (if applicable). Manufacturing activities performed at the site. Products handled (human, veterinary, APIs, biologicals,...

by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Preparation for GMP Audit in Pharmaceuticals 1. Understand the Audit Scope Know whether it’s regulatory (FDA, EMA, MHRA, WHO, CDSCO), customer, or internal. Review previous audit/inspection reports and ensure CAPAs are implemented. Be aware of current guidelines,...
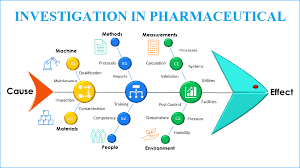
by Dr. Yashashwini Reddy | Sep 9, 2025
🐟 Fishbone Tool of Investigation in Pharmaceuticals 📌 What is It? A cause-and-effect diagram shaped like a fish skeleton. “Head” = problem statement (e.g., OOS result, contamination, deviation). “Bones” = major categories of potential causes. Helps investigation teams...
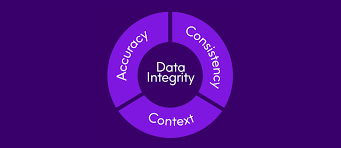
by Dr. Yashashwini Reddy | Sep 9, 2025
🔐 Why Data Integrity is More Important Than Ever? 1. Patient Safety at the Core Medicines are only as safe as the data proving their quality. Any falsified, incomplete, or inaccurate record may lead to unsafe products reaching patients. Strong data integrity ensures...

by Dr. Yashashwini Reddy | Sep 9, 2025
🧫 Typical Microbiology Concerns in an FDA Inspection 1. Environmental Monitoring (EM) Deficiencies Inadequate EM program for cleanrooms and controlled areas. Failure to establish alert/action limits based on historical data. Poor trending and lack of investigation of...







