
by Dr. Yashashwini Reddy | Oct 9, 2025
In pharmaceuticals, specifications are a set of standards, tests, analytical procedures, and acceptance criteria that define the quality requirements for materials and products. They ensure that every product consistently meets its intended safety, efficacy, and...
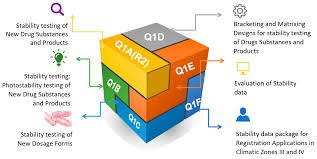
by Dr. Yashashwini Reddy | Oct 8, 2025
📘 ICH Q1: Stability Testing 🔹 Purpose The ICH Q1 series provides guidance on how to design stability studies to ensure that drug substances and drug products maintain their quality, safety, and efficacy throughout their shelf life under various environmental...

by Dr. Yashashwini Reddy | Oct 8, 2025
🧭 Quality Guidelines – Overview Quality Guidelines are internationally harmonized standards developed mainly by the International Council for Harmonisation (ICH) and other regulatory authorities (like FDA, EMA, WHO, CDSCO).They help ensure that pharmaceutical products...

by Dr. Yashashwini Reddy | Sep 29, 2025
In the pharmaceutical industry, quality audits are critical for ensuring compliance with Good Manufacturing Practices (GMP), regulatory guidelines, and internal quality standards. The main types of audits include: Types of Quality Audits in Pharma Internal Audit...

by Dr. Yashashwini Reddy | Sep 29, 2025
Aspect Audit Inspection Definition A systematic, independent review to assess compliance with internal or external standards (e.g., GMP). A formal review by a regulatory authority (e.g., FDA, EMA) to ensure compliance with laws and regulations. Conducted By Internal...







