
by Dr. Yashashwini Reddy | Aug 11, 2025
Calibration of Dissolution Testing Apparatus in Pharmaceuticals 1. Introduction The dissolution test is a critical quality control method in the pharmaceutical industry, ensuring drug release profiles meet pharmacopoeial requirements. Regular calibration of...

by Dr. Yashashwini Reddy | Aug 10, 2025
Why Dissolution Test Apparatus Calibration with Salicylic Acid Tablets was Stopped Historically, salicylic acid tablets were used as calibration standards for dissolution test apparatus (mainly USP Apparatus 1 & 2) to check performance. However, this practice was...
by Dr. Yashashwini Reddy | Aug 10, 2025
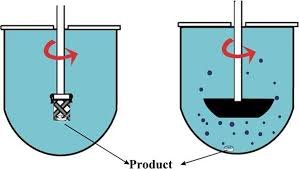
Here’s a clear comparison between Paddle and Basket dissolution methods used in pharmaceutical testing: Parameter Paddle Method (USP Apparatus 2) Basket Method (USP Apparatus 1) Apparatus Rotating paddle fixed at the center above the vessel bottom Rotating cylindrical...

by Dr. Yashashwini Reddy | Aug 9, 2025
Limitations in Dissolution Testing Poor In-Vivo Correlation (IVIVC) Dissolution data may not accurately predict drug release in the human body due to complex gastrointestinal (GI) conditions. Single-Media Testing Conventional USP tests often use only one dissolution...
by Dr. Yashashwini Reddy | May 1, 2025
Standardization and Calibration of Dissolution Test Equipment is a critical aspect of pharmaceutical quality control. It ensures that drug dissolution tests are accurate, consistent, and compliant with regulatory standards such as those set by the United States...







