
by Dr. Yashashwini Reddy | Sep 15, 2025
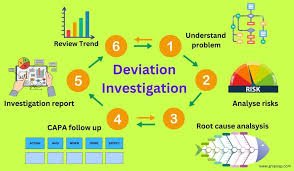
Critical Mistakes During Root Cause Investigation (RCI) Root Cause Investigation (RCI) is an essential step in pharmaceutical quality systems to identify, analyze, and eliminate the underlying causes of deviations, OOS, failures, or non-conformances. However, many...
by Dr. Yashashwini Reddy | Sep 1, 2025
📌 Effective Deviation Management in Pharmaceutical Manufacturing 🔹 What is a Deviation? A Deviation is any departure from approved processes, procedures, specifications, or GMP requirements that may impact product quality, safety, data integrity, or regulatory...
by Dr. Yashashwini Reddy | Aug 12, 2025
Deviation Control in Pharmaceuticals Definition:A deviation is any departure from approved instructions, standard operating procedures (SOPs), specifications, or established Good Manufacturing Practice (GMP) requirements during manufacturing, testing, packaging,...

by Dr. Yashashwini Reddy | Aug 10, 2025
Handling of Out of Calibration (OOC) Instruments and Equipment Definition Out of Calibration (OOC) refers to an instrument or equipment that, during calibration verification, fails to meet the specified acceptance criteria or shows results outside its defined...

by Dr. Yashashwini Reddy | May 7, 2025
Standard Operating Procedure (SOP) 1. Objective To establish standardized procedures for reprocessing and reworking pharmaceutical products to ensure that all batches meet predefined specifications and maintain product quality. 2. Scope...







