
by Dr. Yashashwini Reddy | Sep 15, 2025
📘 Preparation of Annual Product Review (APR) The Annual Product Review (APR) (also referred to as Product Quality Review – PQR in EU) is a regulatory requirement under 21 CFR 211.180(e) and ICH Q7/Q10. Its purpose is to ensure product quality, process consistency, and...

by Dr. Yashashwini Reddy | Sep 15, 2025
Self-Inspection and Quality Audits in Pharmaceuticals 1. Self-Inspection Definition: An internal examination carried out by the company itself to evaluate compliance with GMP, SOPs, and regulatory standards. Purpose: Detect deficiencies in the quality system. Verify...

by Dr. Yashashwini Reddy | Sep 15, 2025
Annual Product Quality Review (APQR/APR/PQR) in Quality Improvements 1. What is APQR/APR/PQR? Annual Product Quality Review (APQR), also known as Annual Product Review (APR) or Product Quality Review (PQR), is a regulatory requirement under ICH Q7, EU GMP Chapter 1...
by Dr. Yashashwini Reddy | Sep 15, 2025
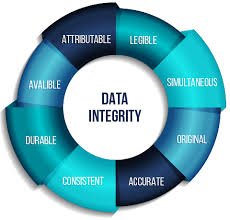
Audit Trail Requirements in Pharmaceuticals An audit trail is a secure, computer-generated, time-stamped record that allows for the reconstruction of events related to the creation, modification, or deletion of electronic data. In the pharmaceutical industry, audit...

by Dr. Yashashwini Reddy | Sep 15, 2025
FDA Forms Generally Used in Pharmaceutical Inspection 1. Form FDA 482 – Notice of Inspection Issued at the start of an inspection. Notifies the company that FDA investigators are officially beginning the inspection under the authority of the FD&C Act (Section...







