
by Dr. Yashashwini Reddy | Sep 6, 2025
Case Studies: Troubleshooting Purified Water System Failures A Purified Water (PW) System must consistently supply water that meets pharmacopeial standards (USP, EP, IP, JP) for use in manufacturing, cleaning, and testing. Failures in the system can lead to OOS...

by Dr. Yashashwini Reddy | Sep 6, 2025
Data Falsification in the Pharmaceutical Industry 1. What is Data Falsification? Data falsification in pharmaceuticals refers to the intentional manipulation, fabrication, or misrepresentation of data in order to conceal deviations, meet specifications, or mislead...
by Dr. Yashashwini Reddy | Sep 6, 2025
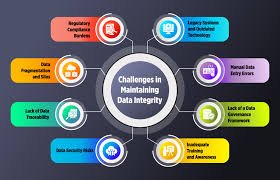
Importance of Data Integrity for Pharmaceutical Regulatory Agencies 1. What is Data Integrity? Data integrity means maintaining the accuracy, completeness, consistency, and reliability of data throughout its lifecycle, ensuring it is attributable, legible,...

by Dr. Yashashwini Reddy | Sep 6, 2025
How to Review GMP Documents Like a Pro 1. Understand the Purpose of the Document Identify the type: SOP, Batch Manufacturing Record (BMR), Protocol, Validation Report, Logbook, etc. Ask: What is this document supposed to control, guide, or prove? 2. Verify Structure...

by Dr. Yashashwini Reddy | Aug 27, 2025
Data Integrity in Microbial Analysis 1. Introduction In microbiology laboratories (especially in pharmaceutical QC/QA), data integrity ensures that all results of microbial testing are accurate, complete, consistent, and trustworthy throughout their lifecycle. Since...







