by Dr. Yashashwini Reddy | Sep 23, 2025
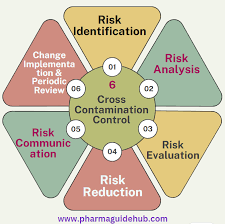
🧪 Case Study: Cross-Contamination in a Multi-Product Pharmaceutical Facility 📌 Background A European pharmaceutical manufacturer operated a multi-product solid oral dosage plant. During a routine EMA inspection, regulatory authorities found traces of a potent API...

by Dr. Yashashwini Reddy | May 2, 2025
Common Sources of Contamination in Pharmaceutical Manufacturing Contamination in pharmaceutical production can lead to serious consequences, including compromised product quality, patient safety risks, regulatory violations, and financial losses. Contamination can be...
by Dr. Yashashwini Reddy | Oct 21, 2024
Identifying the Worst Case in Cleaning Validation In cleaning validation, determining the worst-case scenario is essential to ensure that the cleaning procedures effectively remove the most challenging residues from manufacturing equipment. This scenario represents...





