by Dr. Yashashwini Reddy | Oct 9, 2025
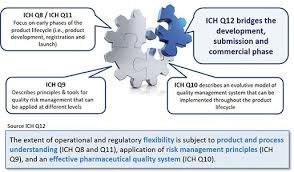
Lifecycle Management (LCM) in the pharmaceutical industry refers to the systematic approach of managing a product from its development phase through commercialization, post-approval changes, and discontinuation. It ensures continuous product quality, compliance, and...
by Dr. Yashashwini Reddy | Oct 9, 2025
A Pharmaceutical Quality System (PQS) is a comprehensive framework that ensures the consistent quality, safety, and efficacy of pharmaceutical products throughout their life cycle — from development to discontinuation. It aligns with ICH Q10, which complements ICH Q8...

by Dr. Yashashwini Reddy | Sep 29, 2025
Formats of Pharmaceutical Audits On-Site Audit (Physical Audit) Conducted at the manufacturing site, laboratory, warehouse, or supplier facility. Provides direct observation of processes, equipment, and personnel. Most common format for GMP compliance checks. Remote...

by Dr. Yashashwini Reddy | Sep 29, 2025
📋 Types of Audits in the Pharmaceutical Industry 1. Internal Audit (Self-Inspection) Purpose: To assess compliance with internal SOPs and GMP requirements. Conducted By: Company’s own QA or compliance team. Frequency: Regularly scheduled (e.g., annually or quarterly)....
by Dr. Yashashwini Reddy | Sep 23, 2025
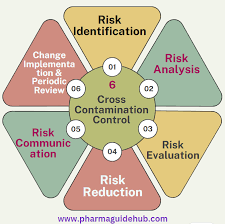
🧪 Case Study: Cross-Contamination in a Multi-Product Pharmaceutical Facility 📌 Background A European pharmaceutical manufacturer operated a multi-product solid oral dosage plant. During a routine EMA inspection, regulatory authorities found traces of a potent API...







