
by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Regulatory Compliance in Pharmaceuticals: 8 Common Mistakes and How to Avoid Them Definition:Regulatory compliance in pharmaceuticals means adhering to laws, regulations, and guidelines (e.g., US FDA, EMA, WHO, MHRA, CDSCO) to ensure medicines are safe, effective,...

by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Contamination Issues in Pharmaceutical Production and Their Prevention Definition:Contamination in pharmaceuticals refers to the undesired introduction of chemical, microbial, or physical material into drug products during manufacturing, packaging, storage, or...
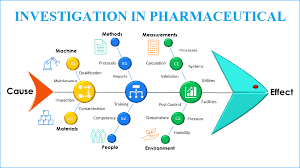
by Dr. Yashashwini Reddy | Sep 9, 2025
🐟 Fishbone Tool of Investigation in Pharmaceuticals 📌 What is It? A cause-and-effect diagram shaped like a fish skeleton. “Head” = problem statement (e.g., OOS result, contamination, deviation). “Bones” = major categories of potential causes. Helps investigation teams...

by Dr. Yashashwini Reddy | Sep 6, 2025
Case Studies: Troubleshooting Purified Water System Failures A Purified Water (PW) System must consistently supply water that meets pharmacopeial standards (USP, EP, IP, JP) for use in manufacturing, cleaning, and testing. Failures in the system can lead to OOS...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To establish a procedure for the operation, cleaning, and maintenance of the Saizoner Mixer Granulator to ensure uniform wet granulation in compliance with cGMP requirements. 2.0 Scope This SOP applies to the Saizoner Mixer Granulator installed in the...







