
by Dr. Yashashwini Reddy | Oct 8, 2025
🧭 Quality Guidelines – Overview Quality Guidelines are internationally harmonized standards developed mainly by the International Council for Harmonisation (ICH) and other regulatory authorities (like FDA, EMA, WHO, CDSCO).They help ensure that pharmaceutical products...
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish guidelines for maintaining personal hygiene of all personnel to ensure product safety, prevent contamination, and maintain a clean working environment. 2. Scope This SOP applies to all employees, contract workers, visitors, and service...
by Dr. Yashashwini Reddy | Sep 18, 2025
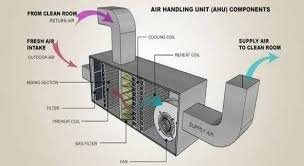
📌 Pharmaceutical AHU and HVAC Components In pharmaceuticals, HVAC (Heating, Ventilation, and Air Conditioning) systems are critical for maintaining controlled environments in cleanrooms, production areas, and laboratories to ensure GMP compliance, product quality, and...
by Dr. Yashashwini Reddy | Sep 18, 2025
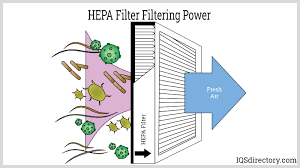
📌 HEPA Filter Uses in Pharmaceutical Manufacturing 🔬 What is a HEPA Filter? High Efficiency Particulate Air (HEPA) Filter removes ≥ 99.97% of airborne particles ≥ 0.3 microns. Essential for cleanroom design and controlled environments. ✅ Uses in Pharmaceutical...

by Dr. Yashashwini Reddy | Sep 18, 2025
📌 Importance of Differential Pressure in Pharmaceuticals Prevents Cross-Contamination Differential pressure ensures controlled airflow between cleanrooms of different classifications. Positive pressure in cleaner areas prevents entry of dust, microbes, and...







