by Dr. Yashashwini Reddy | Sep 8, 2025
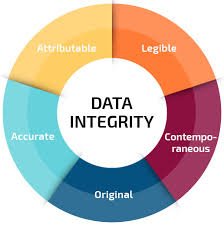
Data Integrity – A Major Problem in Pharmaceuticals Data integrity has become one of the most critical issues in the pharmaceutical industry, directly impacting patient safety, product quality, and regulatory trust. Regulatory agencies like the FDA, EMA, and MHRA...

by Dr. Yashashwini Reddy | Sep 8, 2025
Trends in GMP Violations in Pharmaceuticals Good Manufacturing Practice (GMP) violations remain one of the leading causes of FDA 483s, Warning Letters, and drug recalls. Recent inspection trends show recurring compliance gaps across global pharma companies: 1. Data...

by Dr. Yashashwini Reddy | Sep 6, 2025
Case Studies: Troubleshooting Purified Water System Failures A Purified Water (PW) System must consistently supply water that meets pharmacopeial standards (USP, EP, IP, JP) for use in manufacturing, cleaning, and testing. Failures in the system can lead to OOS...

by Dr. Yashashwini Reddy | Sep 2, 2025
Generic Drugs Manufacturing: Opportunities and Obstacles Opportunities: Cost-effectiveness: Generic drugs offer patients affordable alternatives to branded medicines, increasing accessibility and market demand. Patent Expiry of Blockbuster Drugs: As patents of many...

by Dr. Yashashwini Reddy | Aug 18, 2025
🌟 What Does Quality Really Mean for Pharmaceuticals? In the pharmaceutical industry, quality is far more than just meeting specifications — it is about safeguarding patient health, ensuring regulatory compliance, and maintaining trust. 🔹 Patient Safety First – Every...







