
by Dr. Yashashwini Reddy | Sep 9, 2025
⚠️ 5 Most Common FDA 483 Observations in Pharma 1. Inadequate Investigations (OOS / Deviations / Complaints) Failure to thoroughly investigate out-of-specification (OOS) results, deviations, or complaints. Root cause analysis either incomplete, unjustified, or not...
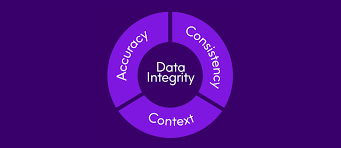
by Dr. Yashashwini Reddy | Sep 9, 2025
🔐 Why Data Integrity is More Important Than Ever? 1. Patient Safety at the Core Medicines are only as safe as the data proving their quality. Any falsified, incomplete, or inaccurate record may lead to unsafe products reaching patients. Strong data integrity ensures...

by Dr. Yashashwini Reddy | Sep 9, 2025
🧫 Typical Microbiology Concerns in an FDA Inspection 1. Environmental Monitoring (EM) Deficiencies Inadequate EM program for cleanrooms and controlled areas. Failure to establish alert/action limits based on historical data. Poor trending and lack of investigation of...

by Dr. Yashashwini Reddy | Sep 8, 2025
Recent GMP Violations at Indian Pharma Facilities 1. Granules India (Telangana) In a 2024 inspection, the FDA observed severe cross-contamination issues: residues in air ducts, microbial contamination despite HEPA filters, bird droppings and feathers in production...
by Dr. Yashashwini Reddy | Sep 8, 2025
Planning and Execution of Internal Audits in Pharmaceuticals Internal audits (self-inspections) are a key part of a pharmaceutical Quality Management System (QMS). They ensure compliance with cGMP, regulatory guidelines, and company SOPs, while also driving continuous...






