
by Dr. Yashashwini Reddy | Sep 15, 2025
Why Data Integrity is More Important Than Ever? Data integrity refers to the completeness, consistency, and accuracy of data throughout its lifecycle. In today’s highly regulated pharmaceutical and healthcare environment, data integrity has become more important than...

by Dr. Yashashwini Reddy | Sep 13, 2025
Liquid Production Audit Checklist 1. Facility & Environment Production area designed as per GMP (segregated zones, unidirectional flow). Cleaning and sanitization records of manufacturing & filling areas available. Air Handling Units (AHUs) qualification and...

by Dr. Yashashwini Reddy | Sep 13, 2025
Warehouse Audit Checklist 1. General Warehouse Practices SOPs available and followed for receipt, storage, dispensing, and distribution. Warehouse access restricted to authorized personnel. Cleanliness and housekeeping maintained as per GMP. Pest control records...
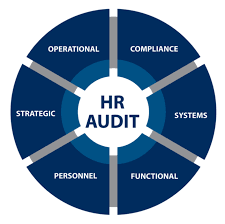
by Dr. Yashashwini Reddy | Sep 13, 2025
HR Audit Checklist 1. Recruitment & Selection Existence of recruitment policies and SOPs. Proper manpower requisition approvals before hiring. Background verification and reference checks of employees. Job descriptions and competency requirements documented....
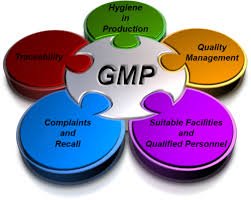
by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Current Good Manufacturing Practices (cGMP) in Pharmaceutical Industries Definition:cGMP (Current Good Manufacturing Practices) are the regulations enforced by regulatory authorities (like US FDA, EMA, WHO, MHRA, CDSCO, etc.) to ensure that pharmaceutical products...







