
by Dr. Yashashwini Reddy | Sep 29, 2025
📋 Types of Audits in the Pharmaceutical Industry 1. Internal Audit (Self-Inspection) Purpose: To assess compliance with internal SOPs and GMP requirements. Conducted By: Company’s own QA or compliance team. Frequency: Regularly scheduled (e.g., annually or quarterly)....
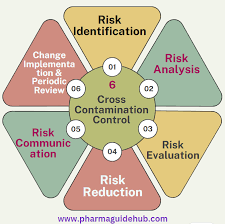
by Dr. Yashashwini Reddy | Sep 23, 2025
🧪 Case Study: Cross-Contamination in a Multi-Product Pharmaceutical Facility 📌 Background A European pharmaceutical manufacturer operated a multi-product solid oral dosage plant. During a routine EMA inspection, regulatory authorities found traces of a potent API...

by Dr. Yashashwini Reddy | Sep 18, 2025
1. Objective Demonstrate that the proposed purified water generation and distribution system is designed, specified and documented such that, when built and operated as designed, it will consistently produce Purified Water that meets USP requirements and is suitable...

by Dr. Yashashwini Reddy | Sep 15, 2025
Why Data Integrity is More Important Than Ever? Data integrity refers to the completeness, consistency, and accuracy of data throughout its lifecycle. In today’s highly regulated pharmaceutical and healthcare environment, data integrity has become more important than...

by Dr. Yashashwini Reddy | Sep 11, 2025
📌 EMA vs FDA Expectations on Process Validation Definition:Process Validation (PV) is the collection and evaluation of data, from process design to commercial production, to establish scientific evidence that a process is capable of consistently delivering quality...







