
by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To lay down the procedure for operation, cleaning, and maintenance of the Cage Blender to ensure uniform mixing of powders and granules in compliance with cGMP. 2.0 Scope This SOP applies to the Cage Blender installed in the production area of [Company...
by Dr. Yashashwini Reddy | Sep 3, 2025
Novel Drug Delivery Systems (NDDS) A Novel Drug Delivery System is a modern approach of formulating and delivering drugs to improve efficacy, safety, patient compliance, and targeted delivery compared to conventional dosage forms (like tablets, capsules, or...

by Dr. Yashashwini Reddy | Sep 3, 2025
Building Better Manufacturing Facilities in Pharmaceuticals Pharmaceutical manufacturing facilities must be designed to ensure product quality, patient safety, and regulatory compliance. A “better” facility goes beyond infrastructure—it integrates technology,...
by Dr. Yashashwini Reddy | Sep 2, 2025
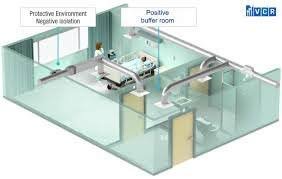
🔹 Buffer Area in Sterile Facility 1. Definition The Buffer Area (also called the cleanroom or critical area) is a Class 100 / ISO 5 / Grade A-B environment where aseptic processing, sterile filling, and direct product exposure operations are performed. It is...

by Dr. Yashashwini Reddy | Sep 2, 2025
1. Influence on Stability Moisture Sensitivity: Smaller granules have a larger surface area, which can increase moisture absorption, leading to hydrolytic degradation of moisture-sensitive drugs. Oxidation: Increased surface area also accelerates oxidative...







