by Dr. Yashashwini Reddy | Sep 15, 2025
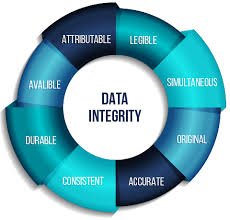
Audit Trail Requirements in Pharmaceuticals An audit trail is a secure, computer-generated, time-stamped record that allows for the reconstruction of events related to the creation, modification, or deletion of electronic data. In the pharmaceutical industry, audit...

by Dr. Yashashwini Reddy | Sep 13, 2025
GMP Audit Checklist – Personnel & Premises 1. Personnel 1.1 Organization & Staffing Adequate number of trained and qualified staff available. Organizational chart available and updated. Clear roles, responsibilities, and reporting lines defined. 1.2 Training...

by Dr. Yashashwini Reddy | Sep 13, 2025
Vendor Audit Checklist 1. General Information Vendor profile and organization structure available. Valid licenses, registrations, and certifications (GMP, ISO, GDP). Regulatory inspection history (FDA, EMA, WHO, local authorities). Change notification procedure in...

by Dr. Yashashwini Reddy | Sep 13, 2025
USFDA Audit Preparation Checklist – Quality Control 1. Laboratory Infrastructure & Environment Lab layout complies with GMP (segregation of microbiology, chemistry, stability). Controlled access to QC labs (only authorized staff). Housekeeping and cleanliness...

by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Data Falsification in the Pharmaceutical Industry Definition:Data falsification is the intentional alteration, manipulation, or fabrication of data in order to misrepresent results and meet regulatory, quality, or business expectations. It is one of the most serious...







